Modeling molecular assembly into chiral nanostructures
Molecular modeling techniques are used to shed light on the assembly of molecules into chiral nanostructures.
The chirality at the supramolecular scale can result from the aggregation of chiral molecules (e.g., in solution) or from the intrinsic symmetry breaking occuring at a surface for an adsorbed molecular layer (be the molecules chiral or not).
Our studies focus on the expression of chirality in the adsorbed monolayers and the transfer of chirality from the molecular elementary units to the supramolecular nanostructures.
Based on the structural information obtained from Molecular Dynamics simulations, it is also possible to calculate the circular dichroism spectra of the chiral supramolecular assemblies.
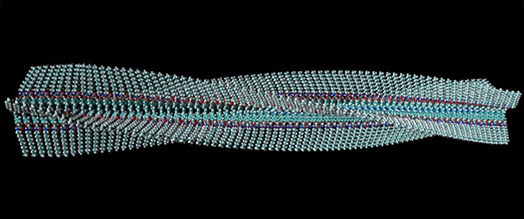
Left: Self-assembly of chiral bis-urea molecules into a helical supramolecular tube (M. Linares et al.)
Right: Helix reversal in a stack of hydrogen-bonded phenylene vinylene oligomers (C. De Leener, B. Van Averbeke et al.)
Assessingthe Role of Chirality in the Formation of Rosette-Like SupramolecularAssemblies on Surfaces.
A.Minoia, Z.Guo, H. Xu, S.J. George, A.P.H.J. Schenning, S. De Feyter, R. Lazzaroni.
Chemical Communications 47 (2011) 10924-10926.
Pasteurian Segregation on a Surface Imaged inSitu at the Molecular Level.
H.Xu, W.J. Saletra, P. Iavicoli, B. Van Averbeke, E. Ghijsens, K.S. Mali,A.P.H.J. Schenning, D. Beljonne, R. Lazzaroni, D.B. Amabilino, S. De Feyter.
Angewandte Chemie 51 (2012) 11981-11985.
Self-Assemblyof Asymmetrically Functionalized [6]Helicene at Liquid/Solid Interfaces“
T.Balandina, M. van der Meijden, O. Ivasenko, D. Cornil, J. Cornil, R. Lazzaroni,R.M. Kellogg, S. De Feyter.
ChemicalCommunications 49 (2013) 2207-2209.
Conformational Plasticity of Hydrogen BondedBis-urea Supramolecular Polymers.
- Brocorens, M. Linares, C. Guyard-Duhayon, R. Guillot, B. Andrioletti, D. Suhr, B. Isare, R. Lazzaroni, L. Bouteiller ; J. Phys. Chem. B, 117 (2013) 5379-5386.
